Valvular heart disease
The four heart valves act as check valves to ensure a organized blood flow throughout the body. The heart valves are exposed to great forces and open and close around 100,000 times a day.
As a result, they can become narrowed (valve stenosis) or leaky (valve insufficiency). Combinations of different heart valve disorders are not uncommon.
Valvular heart disease
The four heart valves act as check valves to ensure a organized blood flow throughout the body. The heart valves are exposed to great forces and open and close around 100,000 times a day.
As a result, they can become narrowed (valve stenosis) or leaky (valve insufficiency). Combinations of different heart valve disorders are not uncommon.
The aortic valve is a non-return valve between the left ventricle and the aorta. It ensures that blood does not flow back into the heart during the filling phase (diastole).
- Shortness of breath, especially during physical exertion.
- Chest pain or pressure, often on exertion or stress.
- Fainting or dizziness and even sudden cardiac death due to reduced blood circulation.
- Strokes
- Signs of late-stage heart failure

- Mostly age-related wear and tear: thickening and/or calcification of the patient’s nativ aortic valve or an aortic valve that has already been replaced (prosthetic stenosis)
- Inflammatory diseases such as rheumatic fever or endocarditis, which lead to stiffening of the cusps
- Long-term effects (> 20-30 years later) after radiation therapy to the chest
- Possible links with elevated lipoprotein (LPa) in research
- Heart auscultation: audible heart murmurs (e.g. systolic murmur) through the narrowed valve.
- Cardiac ultrasound (TTE): for detailed visualization of the valve and the severity of the narrowing as well as the consequences for the heart.
- Cardiac catheterization: To determine the degree of stenosis and to check the coronary arteries.
- Computed tomography from the neck to the pelvis to measure access routes for interventions
- Regular cardiological checks for mild forms
- For severe aortic stenosis, decision on the best procedure in the heart valve team
- Replacement of the aortic valve with a prosthetic valve
- TAVI: Catheter-based procedure on the beating heart (from the age of 75, or in cases of high surgical risk and favorable anatomy)
- SAVR: Surgical replacement of the aortic valve (usually possible via minimally invasive access). For patients <75 years of age or with a high surgical risk, as well as in cases where combined heart surgery is necessary.
- In certain cases, valve-in-valve therapy is also possible (TAVI in SAVR, TAVI in TAVI)
- Medication plays a minor role, purely symptomatic use
- Replacement of the aortic valve with a prosthetic valve
Therapies for aortic valve stenosis:
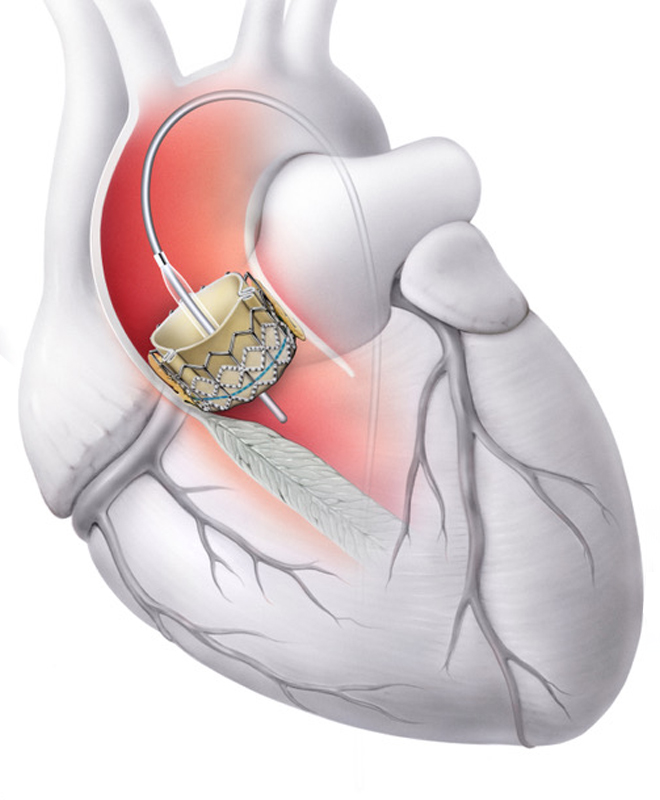
 Transcatheter aortic valve implantation (TAVI) is a minimally invasive procedure for the treatment of severe aortic stenosis. The new valve (a biological valve prosthesis, see left) is advanced to the heart via a catheter, usually through the inguinal artery (transfemoral), and inserted in the area of the diseased valve. The old, calcified valve is not removed, but pressed against the wall by the new prosthesis.
Transcatheter aortic valve implantation (TAVI) is a minimally invasive procedure for the treatment of severe aortic stenosis. The new valve (a biological valve prosthesis, see left) is advanced to the heart via a catheter, usually through the inguinal artery (transfemoral), and inserted in the area of the diseased valve. The old, calcified valve is not removed, but pressed against the wall by the new prosthesis.
The procedure is performed on a beating heart and is carried out under X-ray guidance, sometimes also under ultrasound guidance, to ensure precise placement of the new valve. TAVI is particularly suitable for older patients (>75 years) or those with an increased surgical risk, as it is less stressful than open surgery and allows for a faster recovery. Whether TAVI is possible and advisable depends on various factors and is discussed individually with each patient.
In certain cases, a TAVI can also be performed if the patient’s native aortic valve has already been replaced by a surgical or transcatheter prosthesis and the valve prosthesis has now become stenotic again. The procedure is then called valve-in-valve (TAVI-in-TAVI, TAVI-in-SAVR).
 Surgical aortic valve replacement can be performed either via a classic approach through the sternum (sternotomy) or more gently, minimally invasively through a small incision between the ribs. In both methods, the heart is temporarily stopped and the circulation is taken over by a heart-lung machine. Two types of prosthesis are available to replace the diseased valve: biological valves (made from sterilized pericardial tissue from pigs or cattle) or mechanical valves made from specialized metallic material. Biological valves last on average around 10 years and generally do not require lifelong blood thinning, while mechanical valves are significantly more durable but require a permanent intake of blood thinners. Which access and which valve is chosen depends on various factors and is discussed individually with the patient.
Surgical aortic valve replacement can be performed either via a classic approach through the sternum (sternotomy) or more gently, minimally invasively through a small incision between the ribs. In both methods, the heart is temporarily stopped and the circulation is taken over by a heart-lung machine. Two types of prosthesis are available to replace the diseased valve: biological valves (made from sterilized pericardial tissue from pigs or cattle) or mechanical valves made from specialized metallic material. Biological valves last on average around 10 years and generally do not require lifelong blood thinning, while mechanical valves are significantly more durable but require a permanent intake of blood thinners. Which access and which valve is chosen depends on various factors and is discussed individually with the patient.
- Exertional dyspnea (shortness of breath on exertion)
- Decrease in performance, general fatigue
- Pulsation in the neck (partly visible)
- Heart failure as a long-term consequence
- Prolapse of one or more cusps (prolapse due to connective tissue weakness)
- Degenerative changes with age (native valve) or as a long-term consequence (aortic valve prosthesis)
- Geometric disturbance of the aortic valve due to dilation of the aorta (e.g. caused by high blood pressure or connective tissue diseases)
- Inflammatory processes (e.g. endocarditis, rheumatic fever)
- Congenital valve abnormalities (e.g. bicuspid aortic valve, see chapter on congenital heart disease)
- Auscultation: typical heart murmur
- Cardiac ultrasound (TTE): The most important examination to assess the structure and function of the aortic valve and the heart. Determining the severity of the dysfunction
- Transesophgeal echocardiography (3D TEE): Often used additionally to understand the exact anatomy of the aortic valve
- Cardiac catheterization to measure pressure values in the heart and to visualize the coronary arteries
- CT and/or MRI to visualize the aorta
- Regular cardiological checks for mild forms
- For severe aortic valve insufficiency, decision on the best procedure in the heart valve team
- Medication to relieve the heart (e.g. ACE inhibitors to reduce afterload)
- Surgical or catheter-based valve replacement or surgical valve repair (see below)
- If necessary, combination with replacement of the aortic root or ascending aorta
Therapies for aortic valve regurgitation:
Surgical aortic valve reconstruction is performed using the classic approach through the sternum (sternotomy). In order for the valve to be repaired, the heart is temporarily stopped and the circulation is taken over by the heart-lung machine. The type of reconstruction (retraction, patch implantation…) depends on the underlying valve disease. After successful valve reconstruction, lifelong blood thinning is generally not necessary. Whether aortic valve reconstruction is possible and appropriate depends on various factors and is discussed individually with the patient.
 Surgical aortic valve replacement can be performed either via a classic approach through the sternum (sternotomy) or more gently, minimally invasively through a small incision between the ribs. In both methods, the heart is temporarily stopped and the circulation is taken over by a heart-lung machine. Two types of prosthesis are available to replace the diseased valve: biological valves (made from sterilized pericardial tissue from pigs or cattle) or mechanical valves made from specialized metallic material. Biological valves last on average around 10 years and generally do not require lifelong blood thinning, while mechanical valves are significantly more durable but require a permanent intake of blood thinners. Which access and which valve is chosen depends on various factors and is discussed individually with the patient.
Surgical aortic valve replacement can be performed either via a classic approach through the sternum (sternotomy) or more gently, minimally invasively through a small incision between the ribs. In both methods, the heart is temporarily stopped and the circulation is taken over by a heart-lung machine. Two types of prosthesis are available to replace the diseased valve: biological valves (made from sterilized pericardial tissue from pigs or cattle) or mechanical valves made from specialized metallic material. Biological valves last on average around 10 years and generally do not require lifelong blood thinning, while mechanical valves are significantly more durable but require a permanent intake of blood thinners. Which access and which valve is chosen depends on various factors and is discussed individually with the patient.
 Transcatheter aortic valve implantation (TAVI) is a minimally invasive procedure for the treatment of severe aortic insufficiency. The valve prosthesis (see left) is advanced to the heart via a catheter, usually through the inguinal artery (transfemoral), and inserted in the area of the diseased valve. The old valve is not removed, but pressed against the wall by the new prosthesis.
Transcatheter aortic valve implantation (TAVI) is a minimally invasive procedure for the treatment of severe aortic insufficiency. The valve prosthesis (see left) is advanced to the heart via a catheter, usually through the inguinal artery (transfemoral), and inserted in the area of the diseased valve. The old valve is not removed, but pressed against the wall by the new prosthesis.
The procedure is carried out on the beating heart and is performed under X-ray control, sometimes also under ultrasound control, to ensure precise placement of the new valve. TAVI is an alternative form of treatment, particularly for older patients with an increased surgical risk, as it is less stressful than open surgery and enables a faster recovery. Whether TAVI is possible depends on various factors and is discussed individually with the patient
In certain cases, a TAVI can also be performed if the patient’s native aortic valve has already been replaced by a surgical or transcatheter prosthesis and the valve prosthesis has now become leaky. The procedure is then called valve-in-valve (TAVI-in-TAVI, TAVI-in-SAVR).
The mitral valve is a non-return valve between the left ventricle and the left atrium. It ensures that blood only flows forwards towards the aorta when the heart beats (systole).
- Shortness of breath, especially on exertion
- Cardiac arrhythmia (e.g. atrial fibrillation)
- Reduced performance and rapid exhaustion
- Nocturnal cough or chest pain
- Mostly the result of rheumatic fever (rheumatic heart disease)
- Rare: congenital malformation of the mitral valve
- in old age: due to calcification of the valve or the valve ring
- Long-term effects (> 20-30 years later) after radiation therapy to the chest
- as a long-term consequence after surgical mitral valve replacement (prosthesis stenosis)
- Listening to typical heart murmurs (auscultation)
- Cardiac ultrasound (TTE): The most important examination to assess the structure and function of the mitral valve and the heart. Determining the severity of the dysfunction
- Transesophgeal echocardiography (3D TEE): Often used additionally to understand the exact anatomy of the mitral valve
- ECG/long-term ECG to detect arrhythmias
- Stress tests and cardiac catheterization if necessary
- Decision on the best procedure in the heart valve team
- Drug treatment to alleviate symptoms
- Balloon valvuloplasty (dilatation of the valve using a catheter and balloon) if anatomically possible
- Surgical replacement of the native mitral valve
- Catheter-based replacement for prosthetic stenosis
Therapies for mitral valve stenosis:
Balloon valvuloplasty of the mitral valve is a catheter-based procedure to treat mitral valve stenosis, i.e. a narrowing of the mitral valve. A thin catheter with a small, folded balloon is advanced via the groin vein to the mitral valve. Once the balloon is positioned in the right place, it is carefully inflated to expand the narrowed valve and thus improve blood flow into the left ventricle.
The procedure is performed on the beating heart and under precise imaging control, usually with ultrasound (transesophageal echocardiography) and X-ray. Balloon valvuloplasty is particularly suitable for patients with a certain form of mitral valve stenosis (usually of rheumatic origin) and is an option if surgery would be too risky. In many cases, it can delay the need for valve replacement. Whether balloon valvuloplasty is feasible and appropriate depends on various factors and is discussed individually with the patient.
Catheter-based mitral valve replacement is a new, minimally invasive procedure in which the mitral valve is replaced via a catheter and not surgically via open heart surgery. This method is currently still rarely used and is primarily used in clinical studies and research protocols, as long-term experience in larger patient populations is still lacking.
The Tendyne prosthesis is approved in Switzerland for percutaneous mitral valve replacement. The Tendyne valve is inserted directly into the heart through a small incision at the apex of the heart and anchored in place. In addition, there is a new system, the Sapien M3 prosthesis, which has already been approved in Europe. The Sapien M3 can be advanced to the heart via the vena cava using a transfemoral approach, which is particularly gentle, and inserted into the mitral valve. Both procedures are performed on the beating heart and are carried out under continuous imaging (X-ray and transesophageal echocardiography). Whether percutaneous mitral valve replacement is possible and advisable depends on various factors and is discussed individually with each patient.
Surgical mitral valve replacement can be performed either via a classic approach through the sternum (sternotomy) or more gently, minimally invasively through a small incision between the ribs. In both methods, the heart is temporarily stopped and the circulation is taken over by a heart-lung machine. Two types of prosthesis are available to replace the diseased valve (see left): biological valves (made from sterilized pericardial tissue from pigs or cattle) or mechanical valves made from specialized metallic material. Biological valves last on average around 10 years and generally do not require lifelong blood thinning, while mechanical valves are significantly more durable but require a permanent intake of blood thinners. Which access and which valve is chosen depends on various factors and is discussed individually with the patient.
- Shortness of breath, especially during physical exertion.
- Tiredness and rapid exhaustion (loss of performance).
- Palpitations or irregular heartbeat.
- Signs of late-stage heart failure
- Primary cause: Problem with the mitral valve:
- Signs of wear and tear (degeneration, for example due to tearing of tendon threads) with age.
- Thickening and prolapse (protrusion) can also occur in young people.
- Valve destruction as a result of infections (endocarditis)
- Secondary cause: As a result of other heart diseases (e.g. after a heart attack, in many forms of cheart failure in atrial fibrillation that has persisted for many years)
- Listening to typical heart murmurs (auscultation)
- Cardiac ultrasound (TTE): The most important examination to assess the structure and function of the mitral valve and the heart. Determining the severity of the dysfunction
- Transesophgeal echocardiography (3D TEE): Often used additionally to understand the exact anatomy of the mitral valve
- Cardiac catheterization or coronary CT: Must be performed to exclude additional blocked coronary arteries
- Regular cardiological checks for mild forms
- In cases of severe mitral regurgitation, the heart valve team decides on the best procedure:
- Drug treatment plays a subordinate role.
- In cases of severe and symptomatic mitral regurgitation, the valve is surgically repaired, or replaced if there is no other option (biological or mechanical valve prosthesis)
- In selected cases, especially in cases of high surgical risk / old age, the valve can be repaired using a cardiac catheterization procedure (procedure via the groin, no surgery).
Therapies for mitral regurgitation:

In percutaneous mitral valve repair using the TEER procedure (Transcatheter Edge-to-Edge Repair), the leaking mitral valve is repaired using a catheter on the beating heart without the need to open the chest and use a heart-lung machine. Through a small access in the groin, a catheter is guided via the great vena cava to the mitral valve, where special clips (see left) – either the MitraClip or the PASCAL system – connect the two valve leaflets together and thus reduce the leakage of the valve. The entire procedure is performed under fluoroscopic (X-ray) and echocardiographic (TEE) control so that the exact alignment and positioning of the clips can be visualized in real time. Whether mitral valve reconstruction using TEER is possible and sensible depends on various factors and is discussed individually with the patient.
Catheter-based mitral valve replacement is a new, minimally invasive procedure in which the mitral valve is replaced via a catheter and not surgically via open heart surgery. This method is currently still rarely used and is primarily used in clinical studies and research protocols, as long-term experience in larger patient populations is still lacking.
The Tendyne prosthesis is approved in Switzerland for percutaneous mitral valve replacement. The Tendyne valve is inserted directly into the heart through a small incision at the apex of the heart and anchored in place. In addition, there is a new system, the Sapien M3 prosthesis, which has already been approved in Europe. The Sapien M3 can be advanced to the heart via the vena cava using a transfemoral approach, which is particularly gentle, and inserted into the mitral valve. Both procedures are performed on the beating heart and are carried out under continuous imaging (X-ray and transesophageal echocardiography). Whether percutaneous mitral valve replacement is possible and advisable depends on various factors and is discussed individually with each patient.
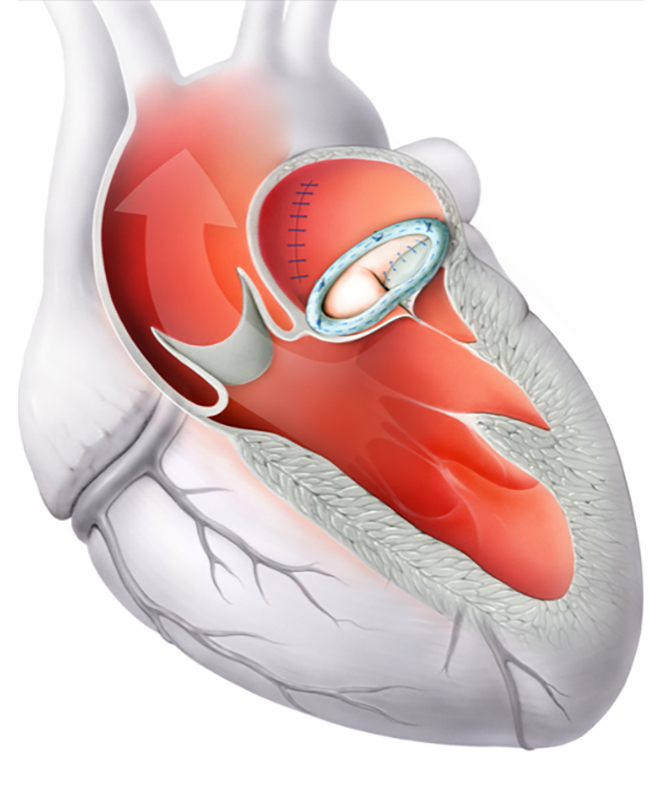 The operation is usually minimally invasive and is performed via a small incision between the right-sided ribs. During the operation, the heart is stopped and the heart-lung machine takes over the circulatory function. In mitral valve reconstruction, the patient’s native valve is repaired so that it opens and closes properly again. Various technical options are available: In almost all cases, a so-called annuloplasty ring (see left) is used to stabilize the valve ring and restore its natural shape. If the chords of the valve are damaged or torn, they can be replaced with artificial chordae. In some cases, small, diseased areas of the valve are surgically removed (resection). The advantages of mitral valve reconstruction are the preservation of the patient’s native valve, the restoration of a natural ring shape (which supports long-term heart function), and, in most cases, the avoidance of lifelong blood thinning. Whether mitral valve reconstruction is possible and advisable, and which approach is chosen, depends on various factors and is discussed individually with the patient.
The operation is usually minimally invasive and is performed via a small incision between the right-sided ribs. During the operation, the heart is stopped and the heart-lung machine takes over the circulatory function. In mitral valve reconstruction, the patient’s native valve is repaired so that it opens and closes properly again. Various technical options are available: In almost all cases, a so-called annuloplasty ring (see left) is used to stabilize the valve ring and restore its natural shape. If the chords of the valve are damaged or torn, they can be replaced with artificial chordae. In some cases, small, diseased areas of the valve are surgically removed (resection). The advantages of mitral valve reconstruction are the preservation of the patient’s native valve, the restoration of a natural ring shape (which supports long-term heart function), and, in most cases, the avoidance of lifelong blood thinning. Whether mitral valve reconstruction is possible and advisable, and which approach is chosen, depends on various factors and is discussed individually with the patient.
Surgical mitral valve replacement can be performed either via a classic approach through the sternum (sternotomy) or more gently, minimally invasively through a small incision between the ribs. In both methods, the heart is temporarily stopped and the circulation is taken over by a heart-lung machine. Two types of prosthesis are available to replace the diseased valve (see left): biological valves (made from sterilized pericardial tissue from pigs or cattle) or mechanical valves made from specialized metallic material. Biological valves last on average around 10 years and generally do not require lifelong blood thinning, while mechanical valves are significantly more durable but require a permanent intake of blood thinners. Which access and which valve is chosen depends on various factors and is discussed individually with the patient.
The tricuspid valve is a non-return valve between the right ventricle and the right atrium. It ensures that blood only flows forwards towards the pulmonary artery when the heart beats. In Switzerland, narrowing (stenosis) of the tricuspid valve is a rarity. However, leakage (insufficiency) of the tricuspid valve is a common clinical problem.
- Leg edema and fluid accumulation in the abdomen (ascites), loss of appetite
- Neck vein distention and visible pulsations of the neck
- Tiredness, shortness of breath and reduced performance
- Secondary (80%)
- Dilation of the right ventricle, often caused by pulmonary hypertension and/or diseases of the left heart
- Dilation of the valve annulus, usually in atrial fibrillation (arrhythmia)
- Primary (20%):
- Congenital or acquired heart valve defects (prolapse)
- Damage due to rheumatic fever or endocarditis
- Mechanical influences, e.g. pacemaker electrodes
- Physical examination, e.g. detection of jugular vein congestion, leg swelling or heart murmur
- Cardiac ultrasound (TTE): The most important examination to assess the structure and function of the tricuspid valve and the heart. Determining the severity of the dysfunction
- Transesophgeal echocardiography (3D TEE): Often used additionally to understand the exact anatomy of the tricuspid valve
- Performance measurement with 6-minute walking test
- Imaging procedures such as MRI or CT for specific questions
- Cardiac catheterization to measure pulmonary vascular resistance
Regular cardiological checks for mild forms
For severe tricuspid valve regurgitation, decision on the best procedure in the heart valve team
- Medication (orally or intravenously): to reduce water retention and to treat heart failure and arrhythmias
- Tricuspid valve reconstruction (surgically or via catheter treatment)
- Tricuspid valve replacement (surgically or via catheter treatment)
Therapies for tricuspid valve regurgitation:
In catheter-based tricuspid valve repair using the TEER procedure (Transcatheter Edge-to-Edge Repair), the leaking tricuspid valve is repaired using a catheter on the beating heart without the need to open the chest and use a heart-lung machine. Through a small access in the groin, a catheter is guided via the great vena cava to the tricuspid valve, where special clips (see left) – either the TriClip or the PASCAL system – connect the valve leaflets to each other and thus reduce the leakage of the valve. The entire procedure is performed under fluoroscopic (X-ray) and echocardiographic (TEE) control so that the exact alignment and positioning of the clips can be visualized in real time. Whether tricuspid valve reconstruction using TEER is possible and appropriate depends on various factors and is discussed individually with the patient.
In addition to the TEER procedure, other catheter-based procedures such as the Cardioband procedure are also used, but have not yet been performed at the Heart Clinic.
Catheter-based tricuspid valve replacement is a new, minimally invasive procedure in which the tricuspid valve is replaced via a catheter and not surgically via open heart surgery. This method is currently still rarely used, as there is a lack of long-term experience in larger patient cohorts.
In Switzerland, the EVOQUE prosthesis is approved for percutaneous tricuspid valve replacement. The prosthesis is advanced to the heart via the great vena cava, i.e. in a particularly gentle manner, and inserted into the tricuspid valve. The procedure is performed on a beating heart and is carried out under continuous imaging (X-ray and transesophageal echocardiography).
If catheter-based valve replacement is not possible due to anatomical reasons or poor function of the right heart, there are other, rarely used methods. In the TricValve® method, for example, biological valve prostheses are inserted into the superior and inferior vena cava to alleviate the symptoms of a severely leaking tricuspid valve.
Whether percutaneous tricuspid valve replacement is feasible and appropriate and which method can be used depends on various factors and is discussed individually with the patient.
The operation is usually minimally invasive and is performed via a small incision between the right-sided ribs. During the operation, the heart is stopped and the heart-lung machine takes over the circulatory function. During tricuspid valve reconstruction, the patient’s native valve is repaired so that it opens and closes properly again. There are various technical options available: It is usually sufficient to insert a so-called annuloplasty ring (see left), which stabilizes the valve ring and restores the natural ring shape. If the chords of the valve are damaged or torn, they can be replaced with artificial chordae. Other techniques are used depending on the valve disease. The advantages of tricuspid valve reconstruction are the preservation of the patient’s native valve, the restoration of a natural annular shape (supports long-term heart function) and, as a rule, the elimination of lifelong blood thinning. Whether tricuspid valve reconstruction is feasible and appropriate and which approach is chosen depends on various factors and is discussed individually with the patient.

Biological valve prosthesis

Mechanical valve prosthesis
Surgical tricuspid valve replacement can be performed either via a classic approach through the sternum (sternotomy) or more gently, minimally invasively through a small incision between the ribs. In both methods, the heart is temporarily stopped and the circulation is taken over by a heart-lung machine. Two types of prosthesis are available to replace the diseased valve (see left): biological valves (made from sterilized pericardial tissue from pigs or cattle) or mechanical valves made from specialized metallic material. Biological valves last on average around 10 years and generally do not require lifelong blood thinning, while mechanical valves are significantly more durable but require a permanent intake of blood thinners. Which access and which valve is chosen depends on various factors and is discussed individually with the patient.
The pulmonary valve is a non-return valve between the right ventricle and the pulmonary artery. It ensures that blood does not flow back into the heart during the filling phase. In healthy adults, there is practically never an isolated severe disorder of the pulmonary valve function. If diseases of the pulmonary valve are present in adulthood, they are almost always the result of a congenital heart defect.
The aortic valve is a non-return valve between the left ventricle and the aorta. It ensures that blood does not flow back into the heart during the filling phase (diastole).
The mitral valve is a non-return valve between the left ventricle and the left atrium. It ensures that blood only flows forwards towards the aorta when the heart beats (systole).
The tricuspid valve is a non-return valve between the right ventricle and the right atrium. It ensures that blood only flows forwards towards the pulmonary artery when the heart beats. In Switzerland, narrowing (stenosis) of the tricuspid valve is a rarity. However, leakage (insufficiency) of the tricuspid valve is a common clinical problem.
The pulmonary valve is a non-return valve between the right ventricle and the pulmonary artery. It ensures that blood does not flow back into the heart during the filling phase. In healthy adults, there is practically never an isolated severe disorder of the pulmonary valve function. If diseases of the pulmonary valve are present in adulthood, they are almost always the result of a congenital heart defect.
Further information
Our specialists for diseases of the heart valves

Prof. Dr. med.
Prof. Dr. med.
Patric Biaggi
Patric Biaggi
Cardiology | Imaging
Cardiology | Imaging
DE – EN – FR – IT
DE – EN – FR – IT

Prof. Dr. med.
Prof. Dr. med.
Roberto Corti
Roberto Corti
Interventional cardiology
Interventional cardiology
DE – FR – IT – EN
DE – FR – IT – EN

Graduate doctor
Graduate doctor
Daniel Fritschi
Daniel Fritschi
Senior physician cardiology
Senior physician cardiology
DE – EN
DE – EN

Prof. Dr. med.
Prof. Dr. med.
Oliver Gämperli
Oliver Gämperli
Interventional cardiology
Interventional cardiology
DE – EN – FR – IT – ES
DE – EN – FR – IT – ES

Prof. Dr. med.
Prof. Dr. med.
Jürg Grünenfelder
Jürg Grünenfelder
Cardiac surgery
Cardiac surgery
DE – EN – IT – FR
DE – EN – IT – FR

Dr. med.
Dr. med.
Ioannis Kapos
Ioannis Kapos
Cardiology | Imaging
Cardiology | Imaging
DE – EN – GR
DE – EN – GR

Prof. Dr. med.
Prof. Dr. med.
Diana Reser
Diana Reser
Cardiac surgery
Cardiac surgery
DE – EN – FR – MA
DE – EN – FR – MA